Until now, electricity from solar energy has been generated and stored in various devices, which is associated with conversion losses. This could soon change: Chemists at FAU Solar and other research institutions in Germany, Australia, the UK, Italy, Sweden and the USA are investigating a hydrocarbon molecule that can either convert sunlight into electricity or store the energy in chemical form over a long period of time. Completely new organic solar modules could be developed on this basis. The basic principles of conversion and storage by the molecule have been published in the ‘Nature Chemistry’.
Solar energy is and remains one of the great hopes of the energy transition. However, because sunlight is a highly volatile energy source, the issue of efficient storage must be resolved. ‘Up to now, we have fed the electricity that we don’t use immediately from the solar module into a battery, from which it is drawn when needed,’ says Prof Dr Julien Bachmann, holder of the Chair of Chemistry of Thin Film Materials (CTFM) at FAU. ‘Due to the multiple changes between chemical and electrical energy, at least 30 per cent of the originally converted energy is lost during battery storage.’
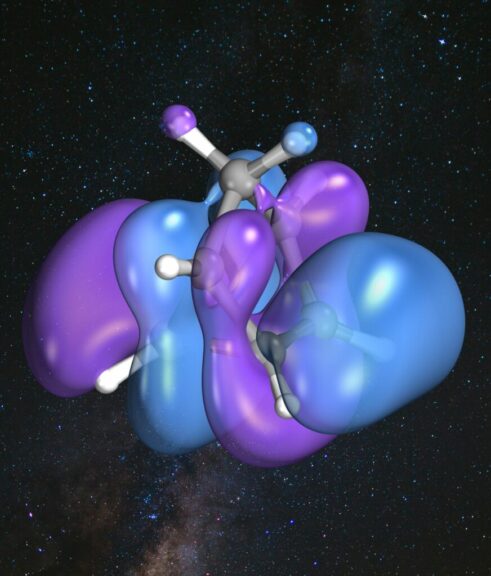
Together with Michael Bosch, a doctoral student at the CTFM department, Bachmann wants to elicit a new property from a familiar material, namely to either convert sunlight into electrical energy or to store it as required. The material in question is norbornadiene, a so-called hydrocarbon isomer consisting of two molecular rings. When norbornadiene is irradiated with ultraviolet light, it transforms into the similarly structured but higher-energy quadricyclane through partial rearrangement of the atomic bonds. ‘The conversion itself is known, but until now research has focussed on recovering the stored energy in the form of heat,’ explains Bachmann. ‘What’s new is that we want to control the process in such a way that the stored energy can now also be utilised as electricity, even months later.’
The physical and chemical basis for the transitions between the isomers is not yet fully understood. In order to change this, researchers from Australia, Great Britain, Italy, Sweden and the USA, together with their FAU colleagues, observed the electron states in a time-resolved manner using photoelectron spectroscopy. Bachmann: ‘The more we know about the dynamics of photo- and electrochemically triggered transformations, the better we can align the molecule design to the desired functions.’ The aim of future research is to utilise not only ultraviolet radiation, but also a broad spectrum of sunlight for electron excitation. ‘The potential is huge,’ explains Julien Bachmann. ‘The pure energy density of the norbornadiene quadricyclane system is comparable to that of a lithium-ion battery.’
If the reversible norbornadiene-quadricyclane conversion can be reliably controlled, it would not only provide an efficient solar module that also stores electricity. The organic, hydrocarbon-based material would also be inexpensive to produce, would not require rare metals and could be disposed of or recycled easily and in an environmentally friendly manner at the end of the product’s life.
Until now, electricity from solar energy has been generated and stored in various devices, which is associated with conversion losses. This could soon change: Chemists at FAU Solar and other research institutions in Germany, Australia, the UK, Italy, Sweden and the USA are investigating a hydrocarbon molecule that can either convert sunlight into electricity or store the energy in chemical form over a long period of time. Completely new organic solar modules could be developed on this basis. The basic principles of conversion and storage by the molecule have been published in the ‘Nature Chemistry’.
Solar energy is and remains one of the great hopes of the energy transition. However, because sunlight is a highly volatile energy source, the issue of efficient storage must be resolved. ‘Up to now, we have fed the electricity that we don’t use immediately from the solar module into a battery, from which it is drawn when needed,’ says Prof Dr Julien Bachmann, holder of the Chair of Chemistry of Thin Film Materials (CTFM) at FAU. ‘Due to the multiple changes between chemical and electrical energy, at least 30 per cent of the originally converted energy is lost during battery storage.’
Together with Michael Bosch, a doctoral student at the CTFM department, Bachmann wants to elicit a new property from a familiar material, namely to either convert sunlight into electrical energy or to store it as required. The material in question is norbornadiene, a so-called hydrocarbon isomer consisting of two molecular rings. When norbornadiene is irradiated with ultraviolet light, it transforms into the similarly structured but higher-energy quadricyclane through partial rearrangement of the atomic bonds. ‘The conversion itself is known, but until now research has focussed on recovering the stored energy in the form of heat,’ explains Bachmann. ‘What’s new is that we want to control the process in such a way that the stored energy can now also be utilised as electricity, even months later.’
The physical and chemical basis for the transitions between the isomers is not yet fully understood. In order to change this, researchers from Australia, Great Britain, Italy, Sweden and the USA, together with their FAU colleagues, observed the electron states in a time-resolved manner using photoelectron spectroscopy. Bachmann: ‘The more we know about the dynamics of photo- and electrochemically triggered transformations, the better we can align the molecule design to the desired functions.’ The aim of future research is to utilise not only ultraviolet radiation, but also a broad spectrum of sunlight for electron excitation. ‘The potential is huge,’ explains Julien Bachmann. ‘The pure energy density of the norbornadiene quadricyclane system is comparable to that of a lithium-ion battery.’
If the reversible norbornadiene-quadricyclane conversion can be reliably controlled, it would not only provide an efficient solar module that also stores electricity. The organic, hydrocarbon-based material would also be inexpensive to produce, would not require rare metals and could be disposed of or recycled easily and in an environmentally friendly manner at the end of the product’s life.