Gotfredsen, H., Thiel, D., Greißel, P. M., Chen, L., Krug, M., Papadopoulos, I., Ferguson, M. J., Nielsen, M. B., Torres, T., Clark, T., Guldi, D. M., Tykwinski, R. R., Journal of the American Chemical Society 2023, 145, 9548-9563. 10.1021/jacs.2c13353
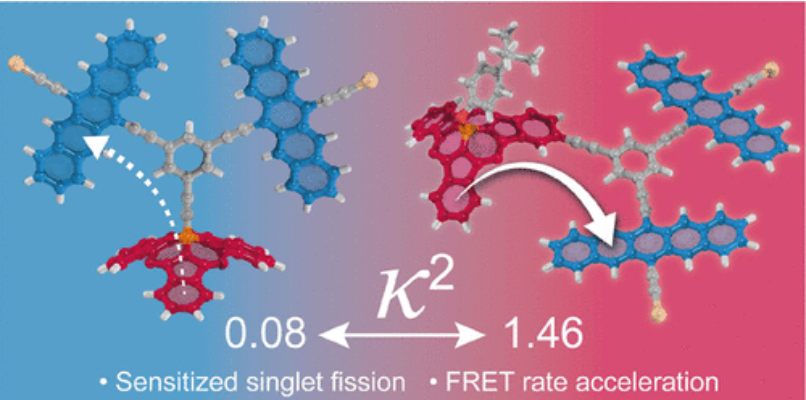
The goal of harnessing the theoretical potential of singlet fission (SF), a process in which one singlet excited state is split into two triplet excited states, has become a central challenge in solar energy research. Covalently linked dimers provide crucial models for understanding the role of chromophore arrangement and coupling in SF. Sensitizers can be integrated into these systems to expand the absorption bandwidth through which SF can be accessed. Here, we define the role of the sensitizer-chromophore geometry in a sensitized SF model system. To this end, two conjugates have been synthesized consisting of a pentacene dimer (SF motif) connected via a rigid alkynyl bridge to a subphthalocyanine (the sensitizer motif) in either an axial or a peripheral arrangement. Steady-state and time-resolved photophysical measurements are used to confirm that both conjugates operate as per design, displaying near unity energy transfer efficiencies and high triplet quantum yields from SF. Decisively, energy transfer between the subphthalocyanine and pentacene dimer occurs ca. 26 times faster in the peripheral conjugate, even though the two chromophores are ca. 3 Å farther apart than in the axial conjugate. Following a theoretical evaluation of the dipolar coupling, Vdip2, and the orientation factor, κ2, of both the axial (Vdip2 = 140 cm–2; κ2 = 0.08) and the peripheral (Vdip2 = 724 cm–2; κ2 = 1.46) arrangements, we establish that this rate acceleration is due to a more favorable (nearly co-planar) relative orientation of the transition dipole moments of the subphthalocyanine and pentacenes in the peripheral constellation.
Gotfredsen, H., Thiel, D., Greißel, P. M., Chen, L., Krug, M., Papadopoulos, I., Ferguson, M. J., Nielsen, M. B., Torres, T., Clark, T., Guldi, D. M., Tykwinski, R. R., Journal of the American Chemical Society 2023, 145, 9548-9563. 10.1021/jacs.2c13353
The goal of harnessing the theoretical potential of singlet fission (SF), a process in which one singlet excited state is split into two triplet excited states, has become a central challenge in solar energy research. Covalently linked dimers provide crucial models for understanding the role of chromophore arrangement and coupling in SF. Sensitizers can be integrated into these systems to expand the absorption bandwidth through which SF can be accessed. Here, we define the role of the sensitizer-chromophore geometry in a sensitized SF model system. To this end, two conjugates have been synthesized consisting of a pentacene dimer (SF motif) connected via a rigid alkynyl bridge to a subphthalocyanine (the sensitizer motif) in either an axial or a peripheral arrangement. Steady-state and time-resolved photophysical measurements are used to confirm that both conjugates operate as per design, displaying near unity energy transfer efficiencies and high triplet quantum yields from SF. Decisively, energy transfer between the subphthalocyanine and pentacene dimer occurs ca. 26 times faster in the peripheral conjugate, even though the two chromophores are ca. 3 Å farther apart than in the axial conjugate. Following a theoretical evaluation of the dipolar coupling, Vdip2, and the orientation factor, κ2, of both the axial (Vdip2 = 140 cm–2; κ2 = 0.08) and the peripheral (Vdip2 = 724 cm–2; κ2 = 1.46) arrangements, we establish that this rate acceleration is due to a more favorable (nearly co-planar) relative orientation of the transition dipole moments of the subphthalocyanine and pentacenes in the peripheral constellation.